Overview
The Lewis structure, a key concept in chemistry, provides important insights into the molecular structure and bonding of different molecules. Comprehending the Lewis structure of the water molecule (H₂O) is essential to appreciating its chemical characteristics, behavior, and importance in scientific and daily situations. Understanding H2O Lewis Structure- This paper explores the creation, meaning, and consequences of the Lewis structure of H2O for a range of scientific and practical applications.
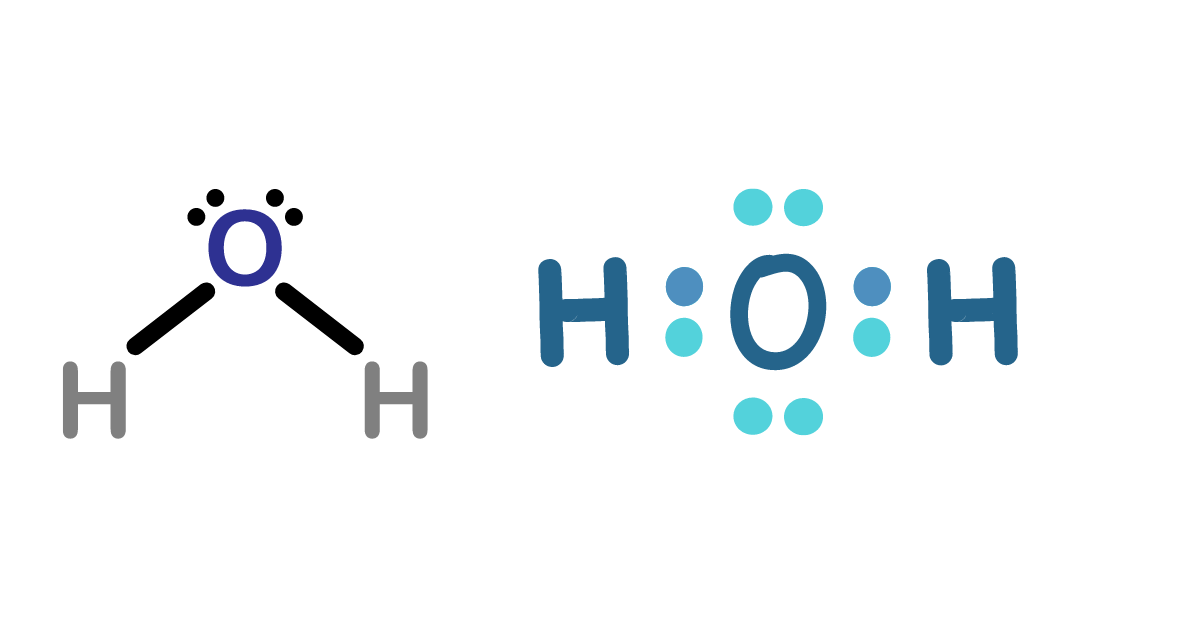
What is the Lewis Structure?-Understanding H2O Lewis Structure
Gilbert N. Lewis, a chemist, created a Lewis structure, a diagram that illustrates the arrangement of atoms, bonds, and valence electrons in a molecule. The structure represents bonds between atoms with lines and valence electrons with dots. Lewis structures are crucial for clarifying chemical bonding, predicting reactivity, and comprehending molecular geometry.
The Significance of Lewis Formations
Lewis structures are useful in chemistry for a number of reasons.
1. Molecular geometry is the study of the three-dimensional arrangement of atoms within molecules.
2. Bonding and Reactivity: They shed light on the interactions between molecules and the bonds that hold atoms together.
3. Predicting qualities: Based on a substance’s molecular structure, they help anticipate its chemical and physical qualities.
The H+O Molecule: A Basic Overview
Water (H2O), one of the most significant molecules on Earth, is known for its unique properties that support life. One oxygen atom and two hydrogen atoms, bound together by a covalent connection, form its composition. Explaining the behavior, characteristics, and function of H2O in many chemical and biological processes requires an understanding of its Lewis structure.
Calculating the Total Valence Electron Count
The total amount of valence electrons accessible for bonding is the first step in developing the Lewis structure of H2O. The outermost electrons of an atom, known as valence electrons, are essential for bond formation.
1. Hydrogen atom (H): Each hydrogen atom contains one valence electron.
2. Oxygen Atom (O): An oxygen atom consists of six valence electrons.
Here’s how to compute the total valence electron count for H2O:
(2 ×1) + 6 = 8 electrons
1. Assign Atoms: Because it is more electronegative, position the oxygen atom in the center and arrange the two hydrogen atoms on either side of it.
2. Draw Bonds: To join each hydrogen atom to the oxygen atom, use a single bond. Each bond contains two electrons.
3. Distribute the remaining electrons: After the bonds form, move the valence electrons of the oxygen atom around. In total, oxygen requires eight electrons, including lone pairs and bonding.
4. Examine the Organization: Make sure that every oxygen atom has a full octet (including bonding and lone pairs), and every hydrogen atom has two electrons (a full valence shell for hydrogen).
The following is an example of H2O’s Lewis structure:
The dots around the oxygen atom in this picture indicate its lone pairs of electrons, whereas each line in the figure indicates a pair of shared electrons.
The Molecular Structure of H₂O
Understanding the molecular geometry of H2O is crucial for understanding its physical and chemical characteristics, and one can achieve this by examining its Lewis structure. The arrangement of the electron pairs in the water molecule gives it a bent or V-shaped geometry.
Molecular geometry and VSEPR theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the form of the H2O molecule based on electron pair repulsion.
1. Electron Pairs Around Oxygen: Oxygen has two lone pairs of electrons and two bonding pairs (with hydrogen).
2. Geometry and Repulsion: The bent molecule shape is the result of electron pairs arranging themselves to reduce repulsion. The molecule takes on a bent form as a result of the lone pairs’ greater repulsion against bonding pairs.

Bond Angles-Understanding H2O Lewis Structure
The H2O molecule’s bond angle is around 104.5 degrees, which is somewhat less than the optimal tetrahedral angle of 109.5 degrees. This decrease is due to the fact that lone pairs repel each other more strongly than bonded pairs do.
The H2O molecule’s polarity
The Lewis structure also explains the polarity of the H2O molecule. To understand how water interacts with other substances, one must understand its polarity and net dipole moment.
Electronegativity and bond polarity
Compared to hydrogen, oxygen is more electronegative, which means that it draws the shared electrons more forcefully. The oxygen atom receives a partial negative charge (δ-) due to the uneven sharing, whereas the hydrogen atom receives a partial positive charge (δ+). This imbalanced distribution of charge gives rise to the dipole moment.
The polarity of molecules
The H2O molecule’s bent form prevents the dipole moments from canceling each other out, creating a net dipole moment. The polarity of water causes numerous peculiar characteristics, such as its high boiling temperature, high surface tension, and solvent capacity.
The Lewis structure in H2O holds great significance.
1. Comprehending the Lewis structure of hydrogen ions is essential for a multitude of scientific and pragmatic uses.
Chemical processes: The Lewis structure can help predict how water will interact with other substances, including whether it will act as a reactant or solvent.
2. Biological activities: Water is necessary for biological activities because of its special qualities, including its capacity to generate hydrogen bonds. These consist of biological processes, enzyme activity, and protein folding.
3. Environmental Science: Knowledge of the H2O molecule facilitates understanding of its behavior in the environment, including its role in the hydrological cycle, weather patterns, and climate.
Typical Myths
Despite the simplicity of the Lewis structure of H2O, there are a few common misconceptions:
1. Inaccurate Bond Angles: People might mistakenly believe that the molecule is linear or that the bond angles in H2O are 90 degrees. The molecule bends, and the correct bond angle is approximately 104.5 degrees.
2. Misunderstanding of Polarity: It is possible to misunderstand the idea of polarity, which might result in inaccurate assumptions regarding the behavior of water. The molecule’s polarity is determined not only by the existence of lone pairs, but also by the bending form and uneven electron sharing.
Further Subjects Associated with H₂O Lewis Structure
Structures of Resonance
Despite the fact that H2O’s Lewis structure is somewhat straightforward, it is important to remember that certain molecules have resonance structures, which allow for the drawing of several Lewis structures. Resonance structures are not necessary for water, though, because its Lewis structure is simple and stable.
Bonding with Hydrogen
Understanding hydrogen bonding also starts with an understanding of H2O’s Lewis structure. Hydrogen bonds weakly attract the hydrogen atoms of two different water molecules to one another. These interactions determine water’s special qualities, such as its high boiling point and surface tension.
H2O and Computational Chemistry
Advanced research can apply computational chemistry techniques to investigate the characteristics of water. Beyond the Lewis structure alone, these techniques often involve intricate computations to predict the behavior of water molecules in various settings and circumstances.
Applications in Practice of Knowing the Lewis Structure of H₂O
1. Water Treatment: To create efficient water treatment procedures, one must first understand the molecular structure of water. The development of filtration and purification techniques benefits from an understanding of the interactions between pollutants and water.
2. Pharmaceuticals: Water is essential to the manufacture of pharmaceuticals. Understanding the characteristics of water simplifies the design of medications and formulations that use it as a solvent or reactant.
3. Agriculture: Understanding water behavior makes it possible to improve agricultural yields through soil moisture management and irrigation technique optimization.
In summary
The Lewis structure of H2O clearly displays the geometry, bonding, and characteristics of the water molecule. It aids in understanding the molecular structure and the elements influencing water’s special properties by displaying the arrangement of atoms and electrons. The Lewis structure of H2O is essential to many scientific fields, from its function in chemical reactions to its importance in biological and environmental activities.
Knowing the Lewis structure of H2O not only improves your understanding of fundamental chemistry, but it also lays the groundwork for learning about more intricate molecular interactions and structures. As you study and apply these ideas, you will gain a better understanding of the complexities of chemistry and the crucial role that molecules like water play in forming our environment. Gaining an understanding of these core ideas will provide you with a strong foundation for learning more about the intriguing field of molecular science.
FAQ:
What is a Lewis structure, and how does it factor into our comprehension of H2O?
A Lewis structure is an illustration of a molecule’s atom, bond, and valence electron arrangements. The Lewis structure depicts the link between oxygen and hydrogen atoms, as well as the sharing of valence electrons in H2O (water). Gaining an understanding of its structure is essential to understanding the molecule’s form, bonding characteristics, and interactions with other materials. Additionally, it helps forecast the behavior of water in a variety of chemical and biological settings.
How would one sketch H3O’s Lewis structure?
Drawing H2O’s Lewis structure:
Calculate the Total Electron Valence Number: Each hydrogen atom has one valence electron, whereas oxygen has six. This equals eight valence electrons for H2O.
Position the Atoms: Arrange the two hydrogen atoms around the oxygen atom, which should be in the middle.
Pull Bonds: Each hydrogen atom and the oxygen atom form a single bond using four electrons, two for each link.
Allocate the Remaining Electrons: Arrange the remaining four electrons in lone pairs surrounding the oxygen atom.
Check the Framework: Verify that every hydrogen atom has two electrons (full valence shell) and every oxygen atom has eight electrons (full octet).
Based on its Lewis structure, what is H2O’s molecular geometry?
H2O has a V-shaped or bent molecule structure. Two bonded pairs and two lone pairs of electrons make up the oxygen atom, according to the Lewis structure. When these electron pairs line up in a way that reduces repulsion, the Valence Shell Electron Pair Repulsion (VSEPR) hypothesis says that they create a bent structure with a bond angle of about 104.5 degrees.
What role does H2O’s Lewis structure play in explaining its polarity?
Because oxygen is more electronegative than hydrogen, there is an uneven distribution of electrons, as seen by the Lewis structure of H2O. The oxygen atom receives a partial negative charge as a result, whereas the hydrogen atom receives a partial positive charge. Because of the bent geometry, which prevents these dipole moments from canceling out, H2O has a net dipole moment, making it a polar molecule. Water’s polarity contributes significantly to its special qualities, including its high boiling point and ability to dissolve various compounds.
How can one use knowledge of H2O’s Lewis structure in real life?
There are several real-world applications for understanding the Lewis structure of H2O.
Water is critical to the design and comprehension of chemical reactions because it helps predict how other chemicals will interact with it.
Understanding the structure of water is helpful in understanding its function in biological processes, including protein folding and enzyme activity.
Environmental Science: Understanding the characteristics of water aids in research into how it behaves in natural settings, including the hydrological cycle and interactions with contaminants.
Water Treatment: By describing how water interacts with pollutants, it helps create efficient techniques for purifying and treating water.



